Overlapping change: Neuroplasticity and Depression
27 Haziran 2025Bükre Naz Nazlı
A complicated psychiatric condition known as major depressive disorder (MDD) is marked by suicidal thoughts, low energy, sleep or eating disturbances, feelings, low self-esteem, and constant sadness. It is a primary cause of disability globally and plays a significant role in the total global illness burden (Rădulescu et al., 2021). The data from the World Health Organization, 264 million individuals worldwide are thought to be affected by MDD. Given that 800,000 individuals die by suicide annually, it is a serious public health problem. MDD negatively changes your feelings, thoughts and your actions while making desicions. Since these effects are directly or indirectly related the brain’s behavioural, sensory, and cognitive activity, MDD and neuroplasticity mechanism of the brain is considered to be interconnected. A mechanism at the systems level, neuroplasticity can include various discrete structural changes that reorganize the brain’s wiring. These changes can include early synaptic plasticity, which can enhance or decrease, spinogenesis, synaptogenesis, synaptic transmission, synapse formation or retraction, the formation of dendrite, axon and their growth and in early developmental stages, unexpectedly neurogenesis (Price & Duman, 2020). The interconnection of MDD with neuroplasticity and the brain revealed in many different approaches. The first approach investigates, with the help of neuroimaging techniques, on observation of hippocampal volume and its visualisation as well as the concentration of brain metabolites under the psychosocial stress among different studies (Malykhin & Coupland, 2015). The second approach investigates on neuroplasticity function in the MDD present brain’s prefrontal cortex and amygdala since it is a wired complex through the brain (Price & Duman, 2020). Another approach focuses on molecular and cellular studies to show the link between MDD and neuroplasticity. This approach centres the regulation of cellular signalling pathways which investigates synapse formation, these studies can be considered related with genetic studies as well, while investigation of molecular biology of MDD through neuroplasticity, some researchers identified genetic characteristics of the concept as well (Pittenger & Duman, 2008). In this research paper all these concepts will be investigated detailly to identify the key points of how does depression affect the ability of neuroplasticity in the brain.
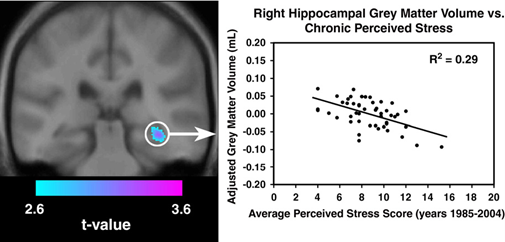
The first approach to the problem of changing neuroplasticity in the brain through MDD is focuses on hippocampus. In MDD research, the hippocampus is the most often examined brain area. From a structural perspective, the hippocampus forms connections with brain areas connected to emotions, such as the prefrontal cortex and amygdala, through nerve fibres which are the building blocks of the limbic system. Furthermore, since it possesses high concentrations of glucocorticoid receptors and glutamate regulates the hypothalamus-pituitary-adrenal (HPA) axis, the hippocampus is particularly susceptible to chronical stress and MDD. The hippocampal plasticity can be changed by stressful situations and other unfavourable inputs. Numerous factors influence the plasticity of the hippocampus. In animal models of MDD, it has been demonstrated that prolonged and intense stress compromises hippocampus-dependent explicit memory (Fuchs et al., 2004). This effect may be explained by changes in the hippocampus synaptic plasticity, as described by long-term MDD (LTD) and long-term potentiation (LTP). It is well acknowledged that the formation of explicit memory, which is based on the hippocampus area, depends on hippocampal synaptic plasticity. High stress levels have been shown in animal studies to worsen LTD and decrease LTP in the hippocampal regions. Stress can also impair neuronal dendritic branching and plasticity in the hippocampus area. Moreover, stress can activate the hypothalamic-pituitary-adrenal axis, increase corticosteroid levels, and decrease hippocampal neurogenesis. When cognitive impairment is present, the levels of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor subunit GluA1 protein in the mouse hippocampus are markedly raised. This can result in MDD in mice models. Furthermore, through tumour necrosis factor receptor 1 signalling, neuropathic pain-induced depressive-like behaviour may be related to neurogeneration in the hippocampus, therefore hippocampal plasticity (Liu et al., 2017). Hippocampal neurogenesis, death of hippocampus neurons, and volumetric alterations in the hippocampus are all components of hippocampal plasticity in MDD. Many reports have indicated that people with MDD have a markedly reduced hippocampus capacity, as shown in Figure 1 (Gianaros et al., 2007). Whether in their first or repeated depressive episode, this condition was observed in adult and adolescent depressed individuals. In female patients with permenant and inherited MDD, the right hippocampus body and tail showed volumetric declines much larger than the left, although total brain volume was about the same as in healthy individuals, according to a recent study. Accordingly, elderly individuals with severe MDD receiving electroconvulsive treatment have been reported to have a notable increase in right hemisphere hippocampus gray matter volume. Nonetheless, those who had recovered from MDD also exhibited a decrease in hippocampus volume. The volumetric alterations in MDD could be caused by mainly high glucocorticoid levels since high glucocorticoid may lead neurodegeneration in the brain. The hippocampus has undergone functional and also structural alterations that are linked to the changes that come from synaptic plasticity by MDD. The disturbance of neurons and glial cells in MDD conditions may lead to the loss in volume of the hippocampal regions. However, the degree of MDD is unrelated to the alterations in hippocampus volume. As solid evidence for these volume changes through changing neuroplasticity, there is a proof which shows people with depression recover more quickly when their hippocampus sizes are greater. These findings can lead the direction of reasoning to the hippocampal regulation of stress reactivity. Reduced hippocampal volumes may be indication of neural damage. Large-sample studies are still required to verify the therapeutic use of hippocampal volumetric changes (Rădulescu et al., 2021)
To deeply understand the mechanism behind the neuroplasticity and depression, the second approach focuses on different parts of the brain than hippocampus. Apart from PFC, several studies have confirmed the morphological and functional alterations in the amygdala, which is linked to MDD. MDD is also linked to the prefrontal cortex (PFC), a key brain region that regulates thought and behavior (Pittenger & Duman, 2008). The prefrontal cortex is separated into two parts in the brain which are the dorsolateral sectors (dlPFC) and the ventromedial prefrontal cortex (vmPFC), based on anatomical connection and functional characteristics. While dlPFC supports cognitive processes including intention formulation, goal-directed activity, and attentional control, VmPFC regulates affection, including the production of unpleasant emotion. It has been demonstrated that both sectors play important roles in MDD. Nevertheless, findings in the literature indicate that their effects exhibit distinct characteristics. Functional imaging studies have revealed opposing changes in activity through two distinct states: hyperactivity in the vmPFC as MDD progresses, i.e., hypoactivity in the dlPFC, while hypoactivity in the vmPFC has been found during recovery with the help of psychotherapy or MDD medication. Moreover, in models that have lesions, vmPFC loss may have a mitigating impact on MDD, while dlPFC loss may exacerbate it. Dysfunction may be brought on by dlPFC injury after a stroke is a risk factor for poststroke MDD. Furthermore, MDD during the early stages of childhood is caused by a reduction in cortical thickness in the right vmPFC, which occurs in the early phases of neurodevelopment (Albert, 2019). In addition to prefrontal cortex, the amygdala is a key brain region for affective modulation and memory encoding. Another important location for neuronal plasticity in fear training is the amygdala. The volume of the amygdala was influenced by the MDD’s degree, in severe MDD cases, the volume changes drastically. Surprisingly, a recent study found that the bilateral amygdalae of first-degree relatives of depressed people exhibited larger gray matter sizes. Furthermore, unpleasant experiences that cause the amygdala to become more active might cause abnormalities in the amygdala, which may be a mild early warning sign for MDD (Liu et al., 2017). According to recent research, MDD can reduce bilateral amygdala-right insular cortex connection and enhance the amygdalar response to stimuli in infants. The latter might stimulate anxiety and MDD symptoms. However, the abnormal functional connections in the MDD left amygdala differ from one another. In the left amygdala, the amygdala positive network’s functional connectivity showing a volume change positively while the amygdala network’s is decreasing. In a clinical study of MDD with early childhood start, there was a reduction in functional link in the bilateral amygdala. Amygdala’s functional link is also impaired in those with late-onset depression. Therefore, a distributed neural network that comprises cortical and limbic parts of the brain rather than a single brain location is responsible for MDD. The lateral prefrontal cortex and the amygdala-associated frontolimbic circuits, and the amygdala-dorsal , and the amygdala-ventromedial prefrontal cortex, which contains also emotional processes, that may exhibit unique dysfunctions in adolescent MDD. The mentioned links may undergo exponential changes in correlation with the severity of MDD and might be used as a biomarker to examine how therapy affects MDD (Fuchs et al., 2004).
Another approach for the interconnection of neuroplasticity and MDD is genetic studies which mostly focuses on gene expression levels. The fact that MDD is thought to be caused by a confluence of environmental, genetically inherited or psychological variables, the brain’s neuroplastic alterations can be considered as possible effectors. Numerous neurotrophic/growth factors, including glial cell-line-derived neurotrophic factor (GDNF) and brain-derived neurotrophic factor (BDNF), have been shown to be important for neuronal plasticity. Additionally, BDNF has a role in the differentiation, proliferation, survival, and migration of neurons in humans. The findings of these studies are supported by research that looks at the mRNA levels of BDNF and mitogen-activated protein kinase (MEK), a starter activator of the BDNF-mediated MEK–ERK pathway, in MDD patients’ and healthy controls’ leukocytes. It’s noteworthy that when compared to control groups the many studies have demonstrated negatively altered mRNA rates in the cell coming from MEK1 and BDNF in depressive patients, which supports the role of MEK1 and BDNF from the presence of MDD (Yang et al., 2020). Furthermore, norepinephrine plays a part in identifying and reacting to stressful circumstances, and it has been proposed that an abnormal norepinephrinergic system may contribute to a heightened susceptibility to MDD. Dopamine is a key neurotransmitter that controls both our desire for rewards and our capacity to experience pleasure. The reason why people with MDD might not appreciate things as much could be explained by decreased dopamine levels. In the neural cells, there are monoamines that controls mood, immunity, energy balance, sleep patterns, and other pathologic elements of MDD to modulate the nervous system as a neurotransmitter. A variety of physiological processes involving the monoamine 5-HT are implicated, and MDD seems to be partially caused by a decrease in serotonin system function. Furthermore, norepinephrine plays a part in identifying and reacting to circumstances that creates stress and anxiety, and it has been proposed that inappropriate functioning of norepinephrinergic system may contribute to a highly possible susceptibility to MDD. One of the important neurotransmitters that regulates our ability to feel pleasure and willing to continue to the life through small happiness such as deleting an item from our to do lists is dopamine. The reason why people with MDD might not appreciate things as much could be explained by decreased dopamine levels. A variety of physiological processes involving the 5-HT are implicated, and MDD seems to be partially caused by a decrease in serotonin system function (Mariani et al., 2021). The aim of these approach is by identifying genes, detect their expressed proteins in order to design antidepressant drugs to target these proteins to rewire the brain with a healthier network of neuroplasticity. Moreover, with the use of several biomarkers to target these MDD-related proteins, the patients can be diagnosed with MDD, therefore the medication can be started immediately without giving any serious conditions to the patients (Pittenger & Duman, 2008).
Consequently, Depression impairs neuroplasticity in the brain by changing neurotransmitter levels, dysregulating the stress response, and impairing synaptic plasticity, all of which compromise the brain’s capacity for adaptation and recuperation. While studies that centres hippocampal neuroplasticity, the neuroplasticity of the other different parts of brain such as prefrontal cortex and amygdala shows changing neuroplasticity under MDD physiologically, genetic studies revealed cellular mechanisms of depression and neuroplasticity. Especially the conceptual genetics studies have opened the door for possible treatments of MDD by altering neuroplasticity ability of the brain via antidepressant agents that targets mutant MDD causing protein expressor genes (Price & Duman, 2020). For the future investigations drug candidates may be investigated.
References:
- Albert, P. R. (2019). Adult neuroplasticity: A new “cure” for major depression? Journal of Psychiatry & Neuroscience : JPN, 44(3), 147. https://doi.org/10.1503/JPN.190072
- Fuchs, E., Czéh, B., Kole, M. H. P., Michaelis, T., & Lucassen, P. J. (2004). Alterations of neuroplasticity in depression: The hippocampus and beyond. European Neuropsychopharmacology, 14(SUPPL. 5). https://doi.org/10.1016/j.euroneuro.2004.09.002
- Gianaros, P. J., Jennings, J. R., Sheu, L. K., Greer, P. J., Kuller, L. H., & Matthews, K. A. (2007). Prospective reports of chronic life stress predict decreased grey matter volume in the hippocampus. NeuroImage, 35(2), 795–803. https://doi.org/10.1016/J.NEUROIMAGE.2006.10.045
- Liu, W., Ge, T., Leng, Y., Pan, Z., Fan, J., Yang, W., & Cui, R. (2017). The Role of Neural Plasticity in Depression: From Hippocampus to Prefrontal Cortex. Neural Plasticity, 2017. https://doi.org/10.1155/2017/6871089
- Malykhin, N. V., & Coupland, N. J. (2015). Hippocampal neuroplasticity in major depressive disorder. In Neuroscience (Vol. 309, pp. 200–213). Elsevier Ltd. https://doi.org/10.1016/j.neuroscience.2015.04.047
- Mariani, N., Cattane, N., Pariante, C., & Cattaneo, A. (2021). Gene expression studies in Depression development and treatment: an overview of the underlying molecular mechanisms and biological processes to identify biomarkers. Translational Psychiatry 2021 11:1, 11(1), 1–23. https://doi.org/10.1038/s41398-021-01469-6
- Pittenger, C., & Duman, R. S. (2008). Stress, depression, and neuroplasticity: A convergence of mechanisms. In Neuropsychopharmacology (Vol. 33, Issue 1, pp. 88–109). https://doi.org/10.1038/sj.npp.1301574
- Price, R. B., & Duman, R. (2020). Neuroplasticity in cognitive and psychological mechanisms of depression: an integrative model. In Molecular Psychiatry (Vol. 25, Issue 3, pp. 530–543). Springer Nature. https://doi.org/10.1038/s41380-019-0615-x
- Rădulescu, I., Drăgoi, A. M., Trifu, S. C., & Cristea, M. B. (2021). Neuroplasticity and depression: Rewiring the brain’s networks through pharmacological therapy (Review). Experimental and Therapeutic Medicine, 22(4). https://doi.org/10.3892/ETM.2021.10565
- Yang, T., Nie, Z., Shu, H., Kuang, Y., Chen, X., Cheng, J., Yu, S., & Liu, H. (2020). The Role of BDNF on Neural Plasticity in Depression. Frontiers in Cellular Neuroscience, 14, 500839. https://doi.org/10.3389/FNCEL.2020.00082/BIBTEX

